Publikationen
Our research
Published:
- T. Haj Hassani Sohi, F. Maass, C. Czekelius, V. Vasylyeva*, A Comparison Study of Roseolumiflavin Solvates: Structural and Energetic Perspective on Their Stability, Crystals 2023, 13(10), 1512. doi: 10.3390/cryst13101512.
- D. Komisarek, F. Demirbas, T. Haj Hassani Sohi, K. Merz, C. Schauerte, V. Vasylyeva*, Special Issue Controlled Crystallization of Active Pharmaceutical Ingredients, 2nd Edition, Polymorphism and Multi-Component Crystal Formation of GABA and Gabapentin, Pharmaceutics 2023, 15(9), 2299. doi 10.3390/pharmaceutics15092299
- T. Heinen, S. Merzenich, A. Kwill, V. Vasylyeva*, Special Issue Covalent and Noncovalent Interactions in Crystal Chemistry II, Halogen Bonding in Sulphonamide Co-Crystals: X···π Preferred over X···O/N? Molecules 2023, 28, 5910. doi: 10.3390/molecules28155910
- D. Komisarek, E. Taskiran, V. Vasylyeva*, Special Issue Advances in Crystal Material: Design, Characterization, and Applications, Maleic Acid as a Co-Former for Pharmaceutically Active GABA Derivatives: Mechanochemistry or Solvent Crystallization? Materials 2023, 16, 2242. doi: 10.3390/ma16062242
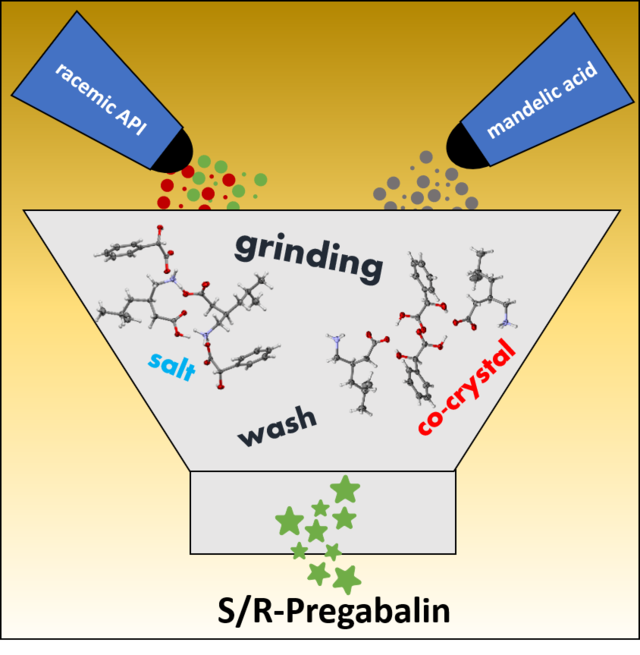
- D. Komisarek, T. Haj Hassani Sohi, V. Vasylyeva*, Back Cover, "Co-Crystals of Zwitterionic GABA API's Pregabalin and Phenibut: Properties and Application", CrystEngComm, 2022, 24, 8390-8398, DOI: 10.1039/D2CE01416E
- M. Herbst, D. Komisarek, T. Strothmann, V. Vasylyeva*, Special issue: Advances in Pharmaceutical Crystals: Control over Nucleation and Polymorphism, "A Lection in Humbleness: Crystallization of Chiral and Zwitterionic APIs Baclofen and Phenibut", Crystals, 2022, 12(10), 1393. https://www.mdpi.com/2073-4352/12/10/1393
- T. Haj Hassani Sohi, F. Maaß, C. Czekelius, M. Suta, V. Vasylyeva*, Special issue: New Talent 2022, "Co-crystallization of organic chromophore roseolumiflavin and effect on its optical characteristics", CrystEngComm, 2022, 24, 7315-7325. DOI: 10.1039/D2CE00589A, https://pubs.rsc.org/en/content/articlelanding/2022/ce/d2ce00589a
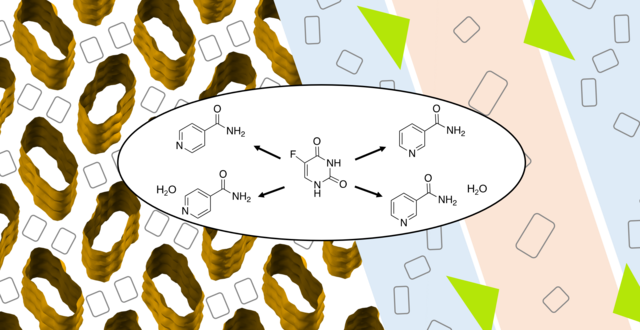
- T. Heinen, S. Hölscher, V. Vasylyeva*, Special Issue: Spotlight on Germany’s Young Crystallographers, " Structural study of anhydrous and hydrated 5-fluorouracil co-crystals with nicotinamide and isonicotinamide", Z. Kristallogr., 2021, 237(4–5), 109–116. https://www.degruyter.com/document/doi/10.1515/zkri-2021-2052/html
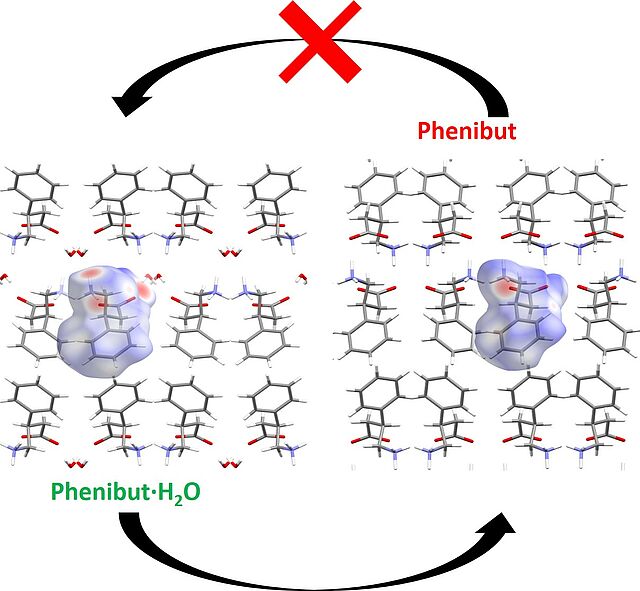
- D. Komisarek, M. Pallaske, V. Vasyleva*, "Crystal Structure and Thermal Properties of Phenibut, Phenibut∙H2O and Phenibut∙HCl: a case for phase stability based on structural considerations", Z. Anorg. Allg. Chem., 2021, 647, 984–991, https://doi.org/10.1002/zaac.202100012
- V. Vasylyeva*, M. Amini, Special issue "In celebration of the 60th birthday of Christoph Janiak", Z. Anorg. Allg. Chem., 2021, 647, 414– 414, https://doi.org/10.1002/zaac.202100067
- O. V. Shishkin, K. Merz, V. Vasylyeva, R. I Zubatyuk, “Isotypic Transformation Principle in Molecular Crystals. Analysis of Supramolecular Architecture of Fluorinated Benzenes and Pyridines”, Cryst. Growth Des., 2018, 18, 8, 4445-4448.
- V. Vasylyeva, L. Catalano, C. Nervi, R. Gobetto, P. Metrangolo, G. Resnati, Issue cover: ”Characteristic redshift and intensity enhancement as far-IR fingerprints of the halogen bond involving aromatic donors”, CrystEngComm, 2016, 18, 2247.
- P. Cerreia Vioglio, L. Catalano, V. Vasylyeva, C. Nervi, M. R. Chierotti, G. Resnati, R. Gobetto, P. Metrangolo, Hot Paper: “Natural Abundance 15N and 13C Solid-State NMR Chemical Shifts:High Sensitivity Probes of the Halogen Bond Geometry”, Chem. Eur. J., 2016, 22, 1.
- V. Vasylyeva, D.W.M. Hofmann, K. Merz, “Crystal structures of 2-chloropyridine and 2-fluoropyridine: Isostructural crystal packing or not?”, Structural Chem., 2016, 27 (1), 331.
- V. Vasylyeva, S. K. Nayak, G. Terraneo, G. Cavallo, P. Metrangolo, G. Resnati, Hot Article: “Orthogonal halogen and hydrogen bonds involving a peptide bond model”, CrystEngComm, 2014, 16, 8102.
- V. Vasylyeva, K. Merz, Special issue on cryo-crystallography, issue cover: “Crystal architecture of the low melting nitrogen heterocycles tetrafluoropyrimidine and trifluorotriazine”, Z. Kristallogr.- Cryst. Mat., 2014, 229 (9), 603.
- O. V. Shishkin, S. V. Shishkina, A. V. Maleev, R. I Zubatyuk, V. Vasylyeva, K. Merz, “Influence of deuteration and fluorination on the supramolecular architecture of pyridine-N-oxide crystals”, ChemPhysChem, 2013, 14(4), 847.
- V. Vasylyeva, O. V. Shishkin, A. Maleev, K. Merz, “Crystal structures of fluorinated pyridines from geometrical and energetic perspectives” Cryst. Growth Des., 2012, 12, 1032.
- A. Kupka, V. Vasylyeva, K. Yusenko. D. W. M. Hofmann, K. Merz, „Solvent and isotopic effects on acridine and deuterated acridine polymorphism“, Cryst. Growth Des, 2012, 12, 5966.
- K. Merz, V. Vasylyeva, Highlight Article, a Review “Development and boundaries in the field of supramolecular synthons”, CrystEngComm, 2010, 12, 3989.
- V. Vasylyeva, K. Merz, “Aggregation of fluorine-substituted pyridines”, J. Fluorine Chem., 2010, 131, 446.
- V. Vasylyeva, T. Kedziorski, N. Metzler-Nolte, C. Schauerte, K. Merz, „Polymorphism of pyridine-N-oxide and its deuterated analogues“, Cryst. Growth Des., 2010, 10, 4224.
- V. Vasylyeva, K. Merz, “Fluorobenzonitriles: influence of the substitution pattern on melting and crystallization properties”, Cryst. Growth Des., 2010, 10, 4250.
Service
- E. Nieland, D. Komisarek, S. Hohloch, K. Wurst, V. Vasylyeva, O. Weingart, B. M. Schmidt, Chem. Commun., 2022, 58, 5233. DOI: 10.1039/d2cc00799a
- A. Karagianni, J. Quodbach, O. Weingart, A. Tsiaxerli, V. Katsanou, V. Vasylyeva, C. Janiak, K. Kachrimanis, Solids 2022, 3, 66–92. https://doi.org/10.3390/solids3010006
- M. Enamullah, V. Vasylyeva, M. A. Quddus, M. K. Islam, S.-P. Höfert, C. Janiak, Issue cover, CrystEngComm, 2018, 20, 4724.
- V. Krieger, E. Ciglia, R. Thoma, V. Vasylyeva, B. Frieg, N. de Sousa Amadeu, T. Kurz, C. Janiak, H. Gohlke, F. K. Hansen, Chem. Eur. J., 2017, 23, 3699.
- M. Enamullah, M. A. Quddus, M. R. Hasan, G. Pescitelli, R. Berardozzi, G. Makhloufi, V. Vasylyeva, C. Janiak, Dalton Trans, 2016, 45, 667.
- M. Enamullah, M. A. Quddus, M. A. Halim, M. K. Islam, V. Vasylyeva, C. Janiak, Inorg. Chim. Acta, 2015, 427, 103.
- R. Bikas, F. Hosseini-Monfared, V. Vasylyeva, J. Sanchiz, J. Alonso, J. M. Barandiaran, C. Janiak, Dalton Trans, 2014, 43, 11925.
- M. Enamullah, A. KM R. Uddin, G. Pescitelli, R. Berardozzi, G. Makhloufi, V. Vasylyeva, A.-C. Chamayou, C. Janiak, Dalton Trans, 2014, 43, 3313.
- C. Heering, I. Boldog, V. Vasylyeva, J. Sanchiz, C. Janiak, CrystEngComm, 2013, 15 (45), 9757.
- G. S. Yellol, A. Donaire, J. G. Yellol, V. Vasylyeva, C. Janiak, J. Ruiz, Chem. Comm., 2013, 49, 11533.
- M. Enamullah, V. Vasylyeva, C. Janiak, Inorg. Chim. Acta, 2013, 408, 109.
- A. Bhunia, V. Vasylyeva, C. Janiak, Chem. Comm, 2013, 49 (38), 3961.
- D. Rönsberg, A. Debbab, A. Mándi, V. Vasylyeva, P. Böhler, B. Stork, L. Engelke, A. Hamacher, R. Sawadogo, M. Diederich, V. Wray, W. Lin, M. Kassack, C. Janiak, S. Scheu, S. Wesselborg, T. Kurtán, A. H. Aly, P. Proksch, J. Org. Chem., 2013, 78, 12409.
- T. Backer, O. Breunig, M. Valldor, K. Merz, V. Vasylyeva, A.-V. Mudring, Cryst. Growth Des., 2011, 11, 2564.
- M. R. Malik, V. Vasylyeva, K. Merz, N. Metzler-Nolte, M. Saleem, S. Ali, A. A. Isab, K. S. Munawar, S. Ahmad, Inorg. Chimica Acta, 2011, 376, 207.
- S. Nawaz, A. A. Isab, K. Merz, V. Vasylyeva, N. Metzler-Nolte, M. Saleem, S. Ahmad, Polyhedron, 2011, 30, 1502.
- K. Splith, I. Neundorf, W. Hu, H. W. P. N'Dongo, V. Vasylyeva, K. Merz, U. Schatzschneider, Dalton Transactions, 2010, 39, 2536.
- J. Lemke, A. Pinto, P. Niehoff, V. Vasylyeva, N. Metzler-Nolte, Dalton Trans., 2009, 35, 7063.